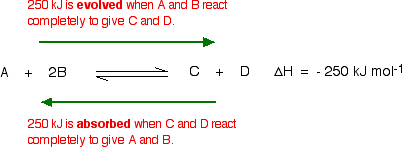
In 1884 the French chemist and engineer Henry-Louis Le Chatelier proposed one of the central concepts of chemical equilibria. Le Chatelier's principle can be stated as follows: A change in one of the variables that describe a system at equilibrium produces a shift in the position of the equilibrium that counteracts the effect of this change.
Le Chatelier's principle describes what happens to a system when something momentarily takes it away from equilibrium. Changing the concentration of one of the components of the reaction
changing the pressure on the system
changing the temperature at which the reaction is run.
No comments:
Post a Comment